Viatris HRT Treatment Navigator
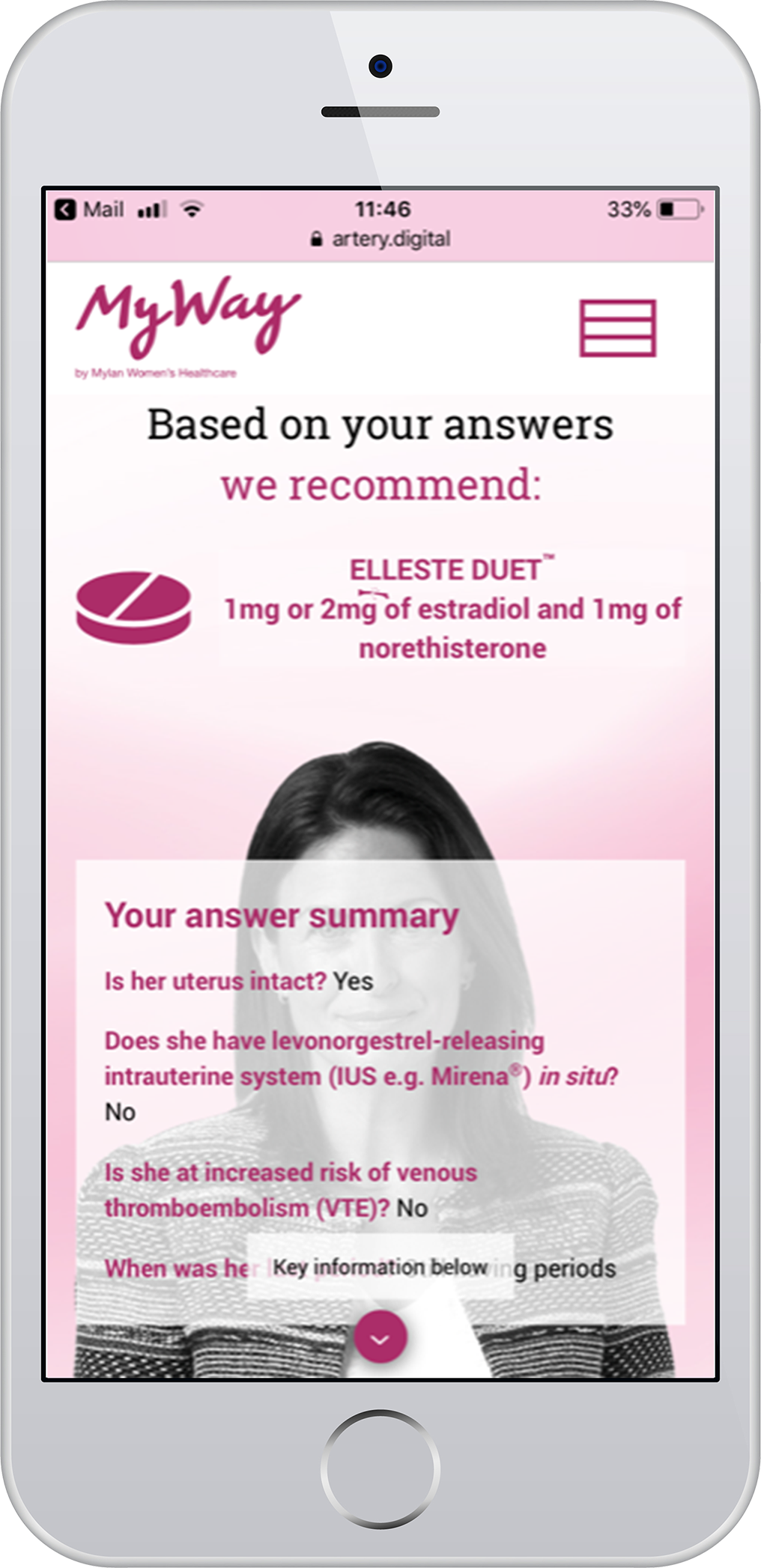
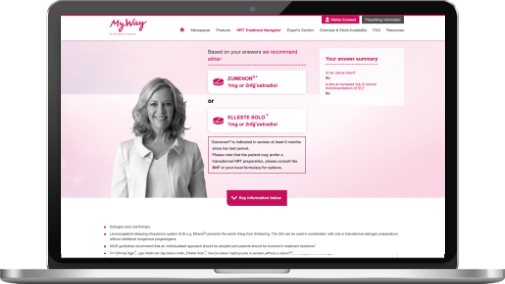
The HRT Treatment Navigator is an online tool designed to aid healthcare professionals to find the most appropriate regime in Viatris' broad range of HRT treatment options.
By answering 2-6 questions, about a women's history, VTE risk factors and previous tolerance, you can find the most suitable option which will come with useful prescriber information.
The Viatris HRT Treatment Navigator is intended to aid and not to replace clinical judgement.
-
References
- 1) NICE Guidelines. Menopause: diagnosis and management. https://www.nice.org.uk/guidance/ng23/resources/menopause-diagnosis-and-management-pdf-1837330217413 (Last accessed September 2021).
- 2) Anderson FA, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003; 107(Suppl. 1): 1-9.
- 3) De Villiers TJ, et al. Updated 2013 International Menopause Society recommendations on menopausal hormone therapy and preventive strategies for midlife health. Climacteric 2013; 16: 316-337.
- 4) MIMS September 2021. www.mims.co.uk
- 5) Elleste Solo™ 1mg Tablets Summary of Product Characteristics.
- 6) Elleste Solo™ 2mg Tablets Summary of Product Characteristics.
- 7) Zumenon® 1mg Summary of Product Characteristics.
- 8) Zumenon® 2mg Summary of Product Characteristics.
- 9) Mirena® Summary of Product Characteristics.
- 10) Utrogestan® Summary of Product Characteristics.
- 11) Elleste Duet™ 1mg Tablets Summary of Product Characteristics.
- 12) Elleste Duet™ 2mg Tablets, Summary of Product Characteristics.
- 13) Schindler AE, Campagnoli C, Druckmann R, et al. Classification and pharmacology of progestins. Maturitas. 2008;61(1–2):171–80.
- 14) Femoston® 1mg/10mg Summary of Product Characteristics.
- 15) Femoston® 2mg/10mg Summary of Product Characteristics.
- 16) Elleste Duet™ Conti Tablets Summary of Product Characteristics.
- 17) Femoston® Conti 0.5mg/2.5mg Summary of Product Characteristics.
- 18) Femoston® Conti 1mg/5mg Summary of Product Characteristics.
- 19) Menopause matters, URL: https://www.menopausematters.co.uk/sideeffects.php (Last accessed September 2021).
- 20) Jones EE. Am J Med 1995; 98(1A): 116S-9S.
- 21) Panay N, et al. Human Reproduction Update 1997; 3( 2): 159-71.
HCP Disclaimer
This website is intended for UK healthcare professionals only.
Viatris Connect is an online platform for UK healthcare professionals.
Across the website you will find news, blogs and product information.
FREE Menopause and HRT webinars available to watch by registering to Viatris Connect today
Please note that the website contains promotional and non-promotional material including educational content and resources to help you and your patients.
REGISTER NOW